Specific heat is a fundamental concept in the field of thermodynamics, referring to the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. This property plays a crucial role in determining how materials respond to thermal energy, affecting their suitability for various applications. Iron, as a widely used material in construction, engineering, and manufacturing, has its specific heat as a key characteristic that influences its behavior in different thermal environments.

✅ AI Essay Writer ✅ AI Detector ✅ Plagchecker ✅ Paraphraser
✅ Summarizer ✅ Citation Generator
The specific heat of iron is particularly important in industries where temperature control and heat management are critical. For example, in the construction of buildings and bridges, understanding the specific heat of iron helps engineers design structures that can withstand temperature fluctuations without compromising their integrity. In manufacturing, knowledge of iron’s specific heat is essential for processes like forging, welding, and heat treatment, ensuring that the material maintains its desired properties under thermal stress.
What is Specific Heat?
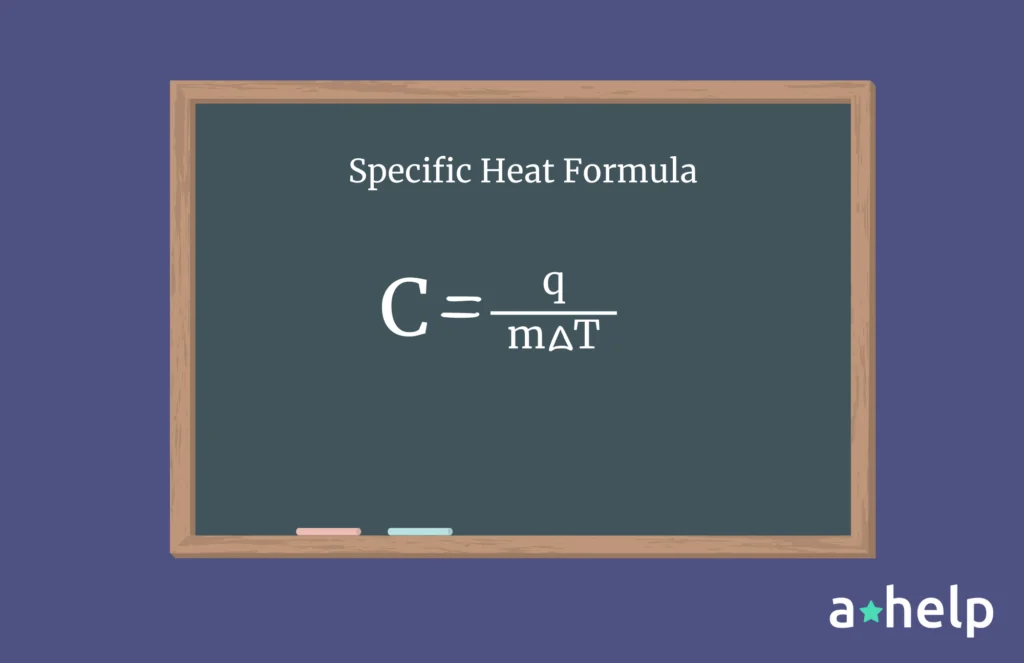
Specific heat capacity, often simply referred to as specific heat, is a fundamental concept in thermodynamics and material science. It is defined as the amount of heat energy required to raise the temperature of one unit of mass of a substance by one degree Celsius (or one Kelvin).
How Is Specific Heat Measured?
To measure the specific heat of a substance, scientists typically use calorimetry. This involves heating a known mass of the substance, measuring the temperature change, and calculating the specific heat using the formula mentioned above. The process requires careful control of conditions and accurate measurement of heat and temperature.
Understanding specific heat is crucial in thermodynamics and material science because it helps predict how substances will behave when they absorb or release heat. For example, materials with high specific heat capacities can absorb a lot of heat without significantly increasing in temperature, making them ideal for applications that require thermal stability, such as heat sinks in electronic devices. Conversely, materials with low specific heat capacities heat up quickly and are used in applications where rapid temperature changes are desired, such as in cooking utensils. The specific heat of a substance is also a key factor in designing heating and cooling systems, as it determines the amount of energy needed to achieve desired temperature changes.
Value of the Specific Heat of Iron and Comparison to Other Materials
The specific heat of iron is a critical property that influences its behavior in various applications. It is the amount of heat required to raise the temperature of one kilogram of iron by one degree Celsius. The specific heat of iron at room temperature is approximately 0.45 J/g°C (joules per gram per degree Celsius). This value can vary slightly depending on the purity and form of the iron.
To put this into perspective, let’s compare the specific heat of iron with that of other common materials:
| 🧪 Material | 🔥 Specific Heat (J/g°C) |
|---|---|
| Iron | 0.45 |
| Aluminum | 0.90 |
| Copper | 0.39 |
| Water | 4.18 |
| Air | 1.01 |
From this table, we can see that iron has a lower specific heat compared to aluminum but higher than copper. This means that iron will heat up faster than aluminum but slower than copper when exposed to the same amount of heat.
The specific heat of iron can be affected by several factors. Temperature is a primary factor; as the temperature of iron increases, its specific heat also increases. This is due to the increased atomic vibrations at higher temperatures, which require more energy to further increase the temperature. Additionally, the purity and alloying elements in iron can influence its specific heat. Impurities and alloying elements can disrupt the lattice structure of iron, affecting its ability to store thermal energy.
Applications of Iron’s Specific Heat
The specific heat of iron plays a vital role in various applications, particularly in construction, engineering, heat treatments, and thermal management.
Iron’s specific heat also plays a significant role in thermal management and energy storage. In systems that require heat dissipation, such as engines and electronic devices, iron components can be used to absorb and transfer heat effectively, thanks to their specific heat capacity. Additionally, iron-based materials are being explored for use in thermal energy storage systems, where their ability to store heat can be harnessed to improve energy efficiency and reduce reliance on fossil fuels.
Conclusion
In conclusion, the specific heat of iron is a fundamental property that has significant implications across multiple disciplines. Its understanding is crucial for the effective application of iron in various industries, and continued research in this area promises to unlock new possibilities and advancements. As we move forward, it is essential to encourage further exploration and application of this knowledge, paving the way for innovative developments that leverage the unique thermal properties of iron.
FAQ
Follow us on Reddit for more insights and updates.




Comments (0)
Welcome to A*Help comments!
We’re all about debate and discussion at A*Help.
We value the diverse opinions of users, so you may find points of view that you don’t agree with. And that’s cool. However, there are certain things we’re not OK with: attempts to manipulate our data in any way, for example, or the posting of discriminative, offensive, hateful, or disparaging material.