Ice floating on water is a common sight, yet it is a phenomenon that puzzles many. This unique behavior of ice is not just a trivial fact; it has profound implications for life on Earth and the natural world. At the heart of this phenomenon is the concept of density, which determines whether a substance will sink or float in another. Unlike most substances, water behaves in an unusual way when it freezes, leading to ice having a lower density than liquid water. Understanding why ice floats is essential not just for scientific projects, but also for getting to know its impact on everyday life, from the simple pleasure of ice cubes in a drink (who doesn’t love their ice latte?) to the survival of aquatic ecosystems during winter.

✅ AI Essay Writer ✅ AI Detector ✅ Plagchecker ✅ Paraphraser
✅ Summarizer ✅ Citation Generator
The Science Behind Ice’s Buoyancy
Density is a measure of how much mass is contained in a given volume. It plays a crucial role in buoyancy, which is the ability of an object to float in a fluid. When an object is placed in water, it will float if its density is less than that of water. In its liquid state, water has a density of approximately 1 gram per cubic centimeter (g/cm³). However, when water freezes to form ice, its density decreases to about 0.92 g/cm³. This is because the molecular structure of ice is different from that of liquid water.
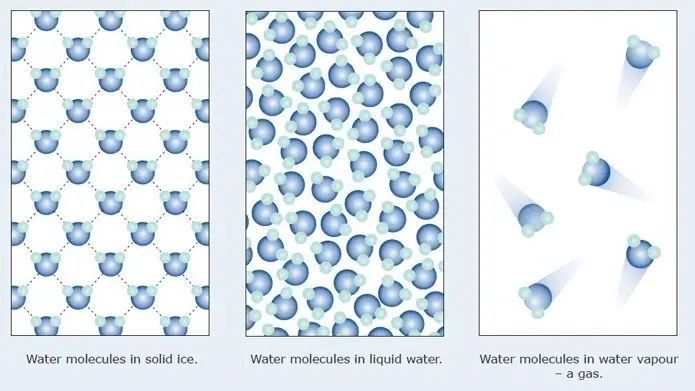
In liquid water, the molecules are closely packed but move freely, allowing them to occupy a smaller volume. In contrast, ice has a crystalline structure where water molecules are arranged in a hexagonal pattern, creating more space between them. This arrangement increases the volume of ice compared to an equal mass of liquid water, leading to a lower density and allowing ice to float.
Here’s a table comparing the properties of liquid water and ice:
| Property | Liquid Water | Ice |
|---|---|---|
| Density | Approximately 1 g/cm³ | Approximately 0.92 g/cm³ |
| Molecular Arrangement | Molecules are closely packed but move freely | Molecules are arranged in a hexagonal pattern, creating more space between them |
| Effect on Buoyancy | Higher density means objects with density less than 1 g/cm³ will float | Lower density allows ice to float on liquid water |
| State of Matter | Liquid | Solid |
| Temperature Range | 0°C to 100°C (at atmospheric pressure) | Below 0°C |
| Response to Pressure | Slightly compressible | Expands when freezing, becoming less dense |
Explaining the Concepts of Density and Buoyancy (Examples)
🏐🏊♂️ Rubber Ball and Rock in a Pool. Imagine a rubber ball and a rock in a swimming pool. The rubber ball is like ice, and the rock is like liquid water. Even though the rubber ball and the rock might be the same size, the rubber ball is lighter and less dense, so it floats on the water’s surface. On the other hand, the rock is heavier and denser, so it sinks to the bottom of the pool. This is similar to how ice, being less dense than liquid water, floats on top of it.
🧽🗿 Sponge and Stone. Think of a sponge and a stone. A sponge is full of tiny holes, making it less dense and allowing it to float in water. A stone, however, is solid and dense, so it sinks. This analogy helps illustrate how the molecular structure of ice, with its spaced-out hexagonal pattern, makes it less dense than liquid water, allowing it to float.
🍿🍬 Popcorn and Candy in a Bowl. Imagine a bowl filled with popcorn and candy. The popcorn represents ice, and the candy represents liquid water. The popcorn is lighter and takes up more space, so it sits on top of the denser, heavier candy at the bottom of the bowl. This is similar to how ice floats on the surface of water.
The Anomalous Expansion of Water
Water exhibits a unique behavior when it freezes, known as the anomalous expansion of water. Unlike most substances that contract and become denser when they solidify, water expands and becomes less dense. This is due to the formation of the hexagonal crystalline structure in ice, which takes up more space than the disordered arrangement of molecules in liquid water.
The maximum density of water occurs at 4°C, where it reaches 1 g/cm³. As water cools below this temperature, it begins to expand, decreasing in density until it freezes. This expansion is crucial for ice formation because it allows ice to be less dense than liquid water, enabling it to float. If water behaved like most other substances and became denser upon freezing, ice would sink, drastically altering the Earth’s climate and the survival of aquatic life.
All in All
In conclusion, the ability of ice to float on water is a result of its unique molecular structure and the anomalous expansion of water upon freezing. This property has far-reaching consequences, from the preservation of aquatic life under frozen lakes to the regulation of Earth’s climate. The floating ice acts as an insulating layer, maintaining a stable environment for organisms below and influencing global temperature patterns.
FAQ
Follow us on Reddit for more insights and updates.



Comments (0)
Welcome to A*Help comments!
We’re all about debate and discussion at A*Help.
We value the diverse opinions of users, so you may find points of view that you don’t agree with. And that’s cool. However, there are certain things we’re not OK with: attempts to manipulate our data in any way, for example, or the posting of discriminative, offensive, hateful, or disparaging material.