An electron is a tiny, incredibly lightweight subatomic particle that carries a negative electric charge. Imagine it as a minuscule speck, much smaller than an atom, zipping around at high speeds. Electrons are essential building blocks of matter, found in everything from the air we breathe to the screen you’re reading this on.

✅ AI Essay Writer ✅ AI Detector ✅ Plagchecker ✅ Paraphraser
✅ Summarizer ✅ Citation Generator
They play a key role in various scientific fields, especially in chemistry and physics. In chemistry, electrons are the glue that holds atoms together in molecules, forming the basis of chemical bonds. In physics, they are crucial for the flow of electricity in circuits and are responsible for the dazzling array of colors in fireworks and neon signs, as they emit light when they move between energy levels in an atom.
Discovery of the Electron
The journey to discovering the electron began in the late 19th century with a scientist named J.J. Thomson (1856-1940). He was experimenting with cathode ray tubes, which are essentially glass tubes that emit a beam of particles when an electric current is passed through them.
Thomson noticed that these beams, called cathode rays, were attracted to positively charged plates, suggesting that they were made of negatively charged particles. He conducted a series of experiments to study these particles’ properties and concluded that they were much smaller than atoms and carried a negative charge. Thomson called these tiny particles “electrons,” a name that has stuck ever since. This groundbreaking discovery challenged the existing atomic theory of the time and paved the way for the development of quantum mechanics in the 20th century, revolutionizing our understanding of the natural world.
Electron Cloud Model
Imagine an atom as a tiny, bustling city, with the nucleus as the city center and electrons as citizens moving around it. In the electron cloud model, these electrons are not confined to specific paths or orbits, like cars on a highway. Instead, they are more like a swarm of bees buzzing around the nucleus, forming a “cloud” of negative charge.
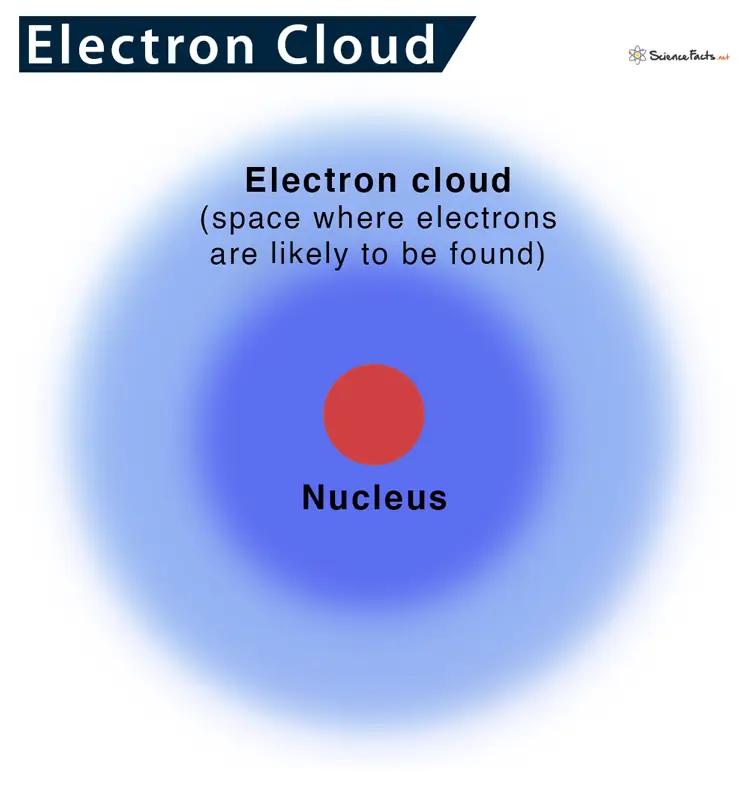
This cloud isn’t uniform; it’s denser in some areas than others. These denser areas indicate where an electron is more likely to be found at any given moment. This model is a leap forward from earlier atomic models because it embraces the unpredictable nature of electrons, as described by quantum mechanics. It gives us a more accurate and dynamic picture of what’s happening inside an atom, showing us that electrons are more about probabilities than certainties.
The Role of Electron Cloud in Physics and Chemistry
The electron cloud model is like a key that unlocks many mysteries in physics and chemistry. In physics, it helps us understand how atoms and molecules interact with each other and with light. It explains why some materials conduct electricity while others don’t and why some substances are solid, others are liquid, and yet others are gas. In chemistry, the electron cloud model is essential for understanding how atoms bond together to form molecules. It shows us that chemical bonds are all about electrons being shared or transferred between atoms. This model also helps us predict how substances will react with each other, which is crucial for everything from creating new medicines to developing environmentally friendly energy sources. In short, the electron cloud model is a fundamental concept that helps us understand the behavior of matter at its most basic level.
Conclusion
The electron cloud model represents a significant advancement in our understanding of atomic structure. It has replaced earlier models that depicted electrons in fixed orbits, offering a more accurate and comprehensive view of the electron’s behavior. This model is not only a cornerstone of quantum mechanics but also a fundamental concept in modern chemistry and physics. It underscores the importance of electrons in shaping the physical and chemical properties of matter and continues to be a vital tool in scientific research and discovery.
FAQ
Follow us on Reddit for more insights and updates.





Comments (0)
Welcome to A*Help comments!
We’re all about debate and discussion at A*Help.
We value the diverse opinions of users, so you may find points of view that you don’t agree with. And that’s cool. However, there are certain things we’re not OK with: attempts to manipulate our data in any way, for example, or the posting of discriminative, offensive, hateful, or disparaging material.